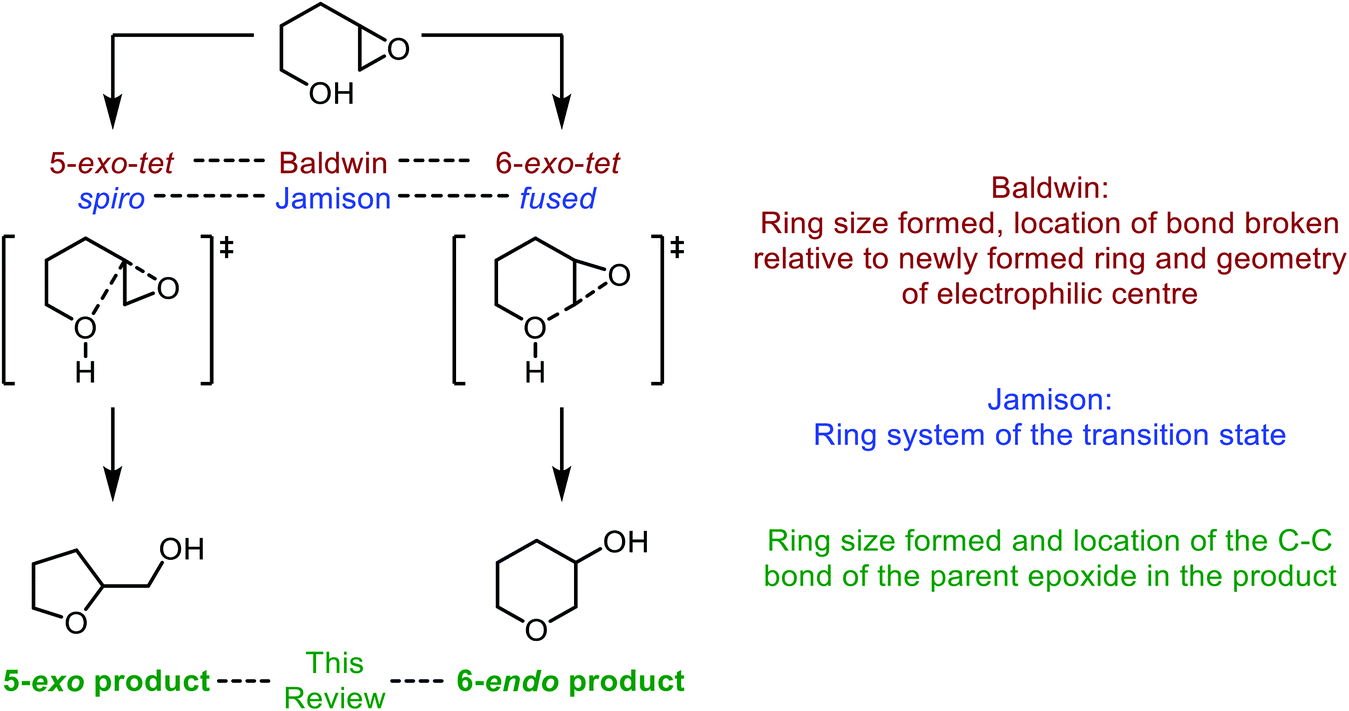
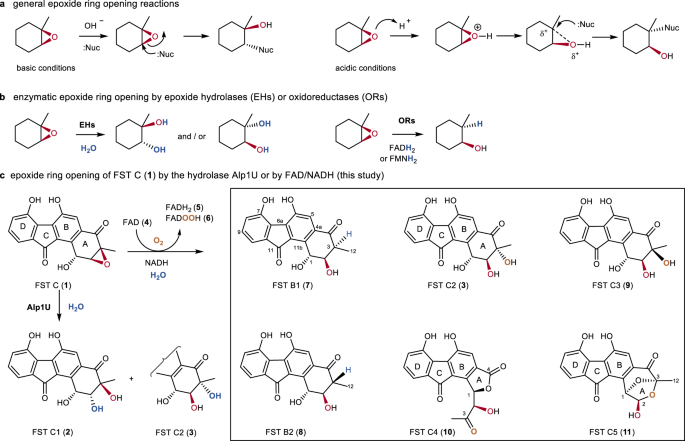
Mechanism and kinetics of epoxide ring-opening with carboxylic acids catalyzed by the corresponding carboxylates - ScienceDirect

Scheme 2. Computationally analyzed ring-opening reactions of epoxide 1... | Download Scientific Diagram

Brønsted Acid‐Catalysed Epoxide Ring‐Opening Using Amine Nucleophiles: A Facile Access to β‐Amino Alcohols - Tyagi - 2022 - Chemistry – An Asian Journal - Wiley Online Library

Mechanism and kinetics of epoxide ring-opening with carboxylic acids catalyzed by the corresponding carboxylates - ScienceDirect

Catalytic reductive ring opening of epoxides enabled by zirconocene and photoredox catalysis - ScienceDirect

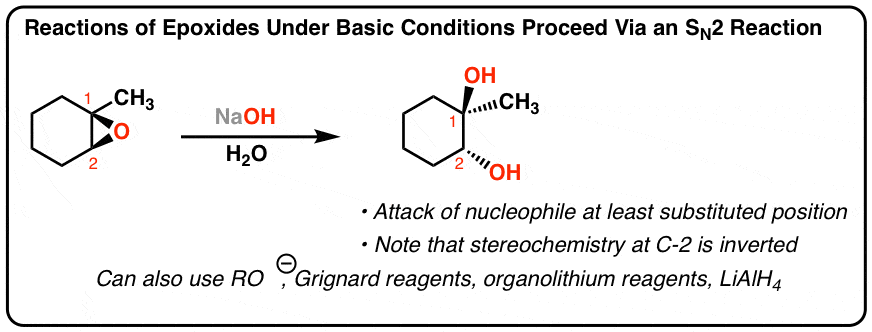
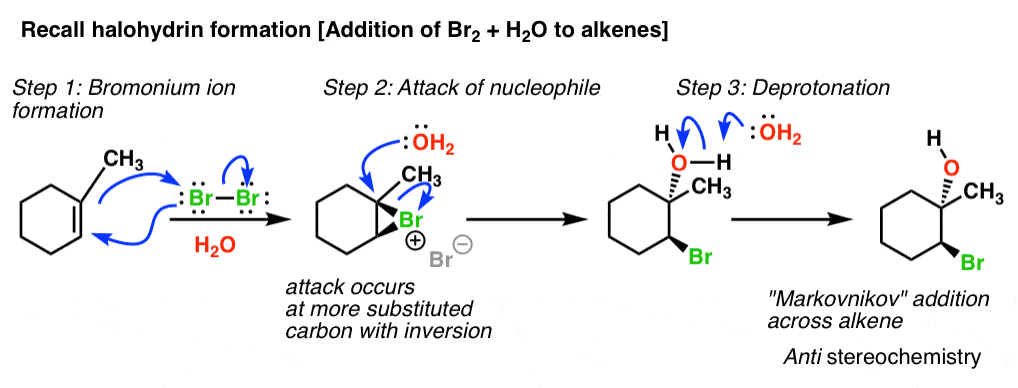
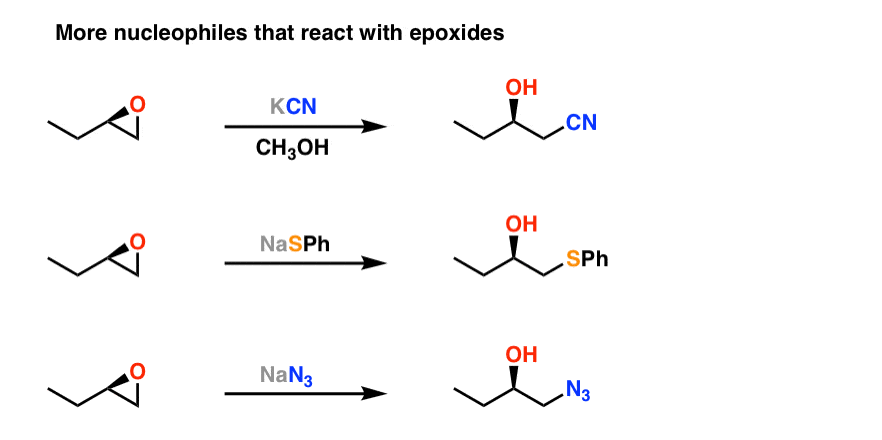
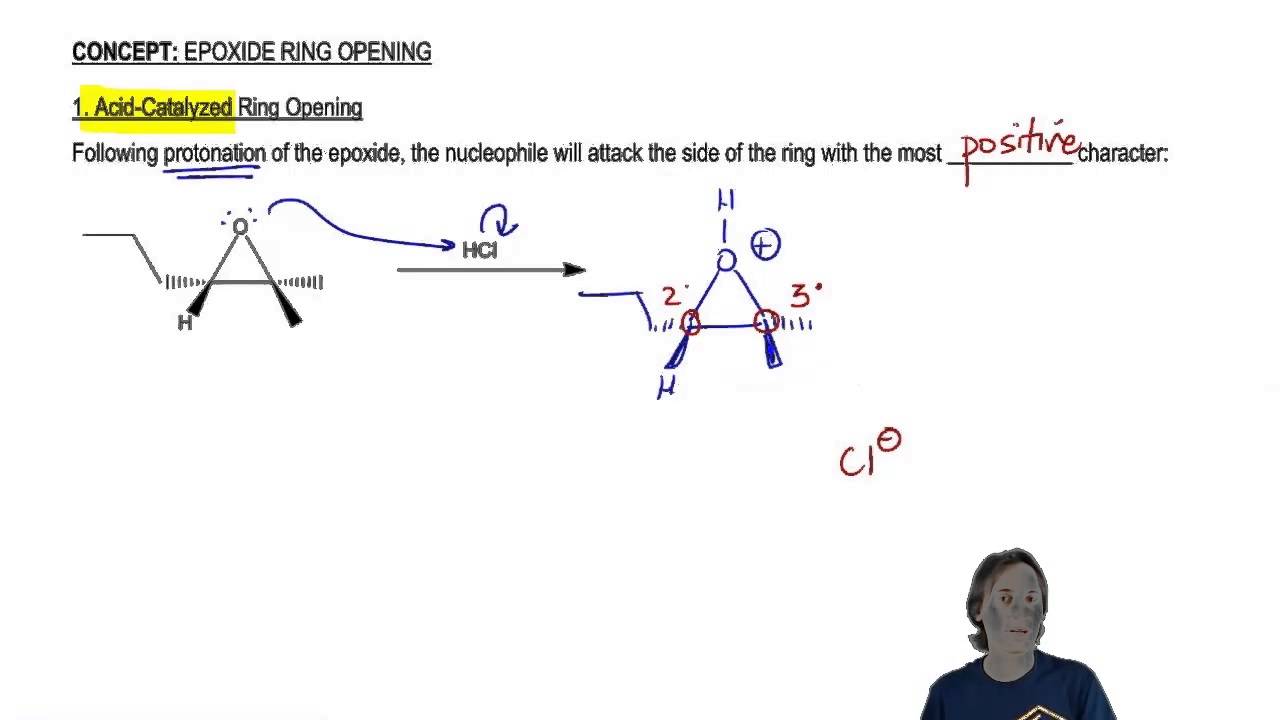
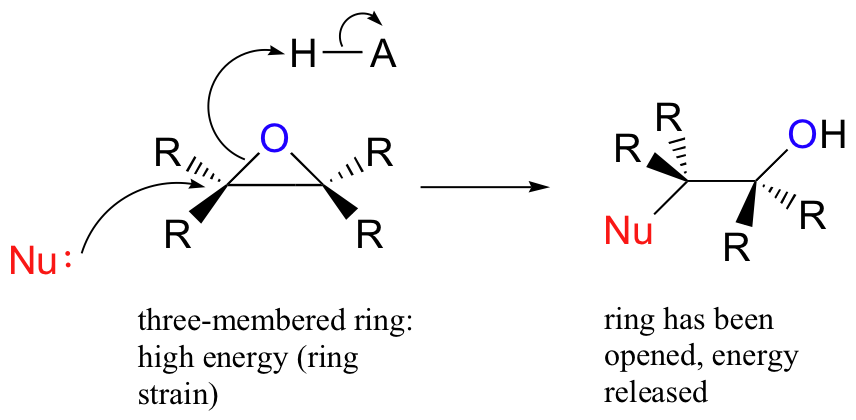
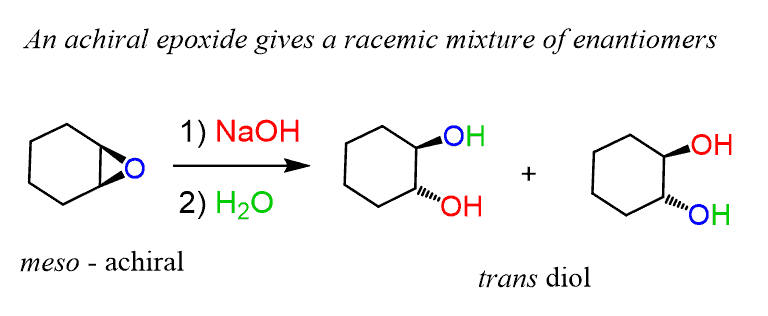
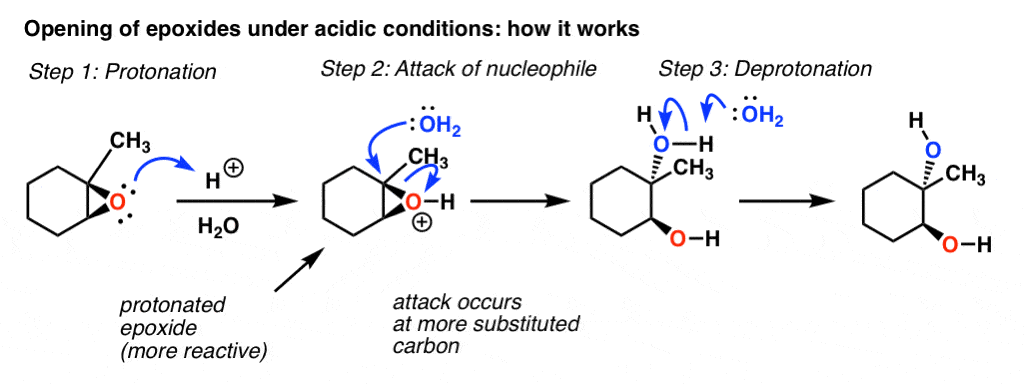
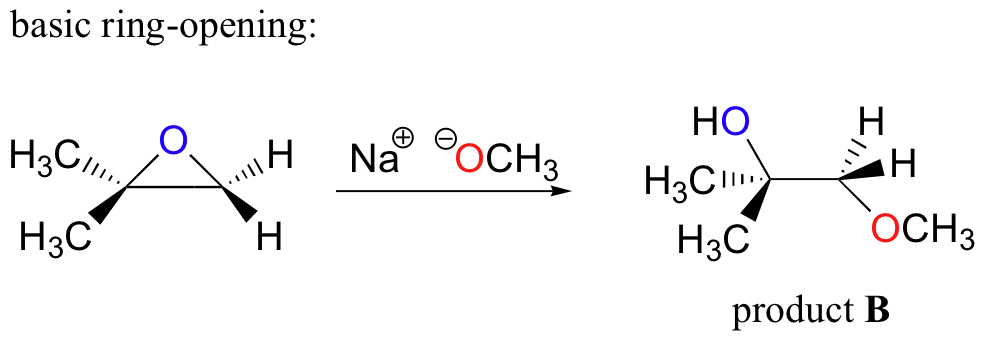
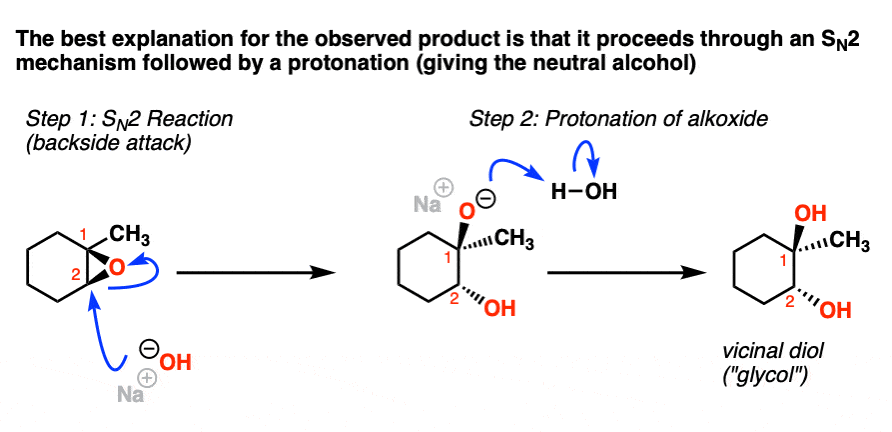
Regioselectivity of Epoxide Ring‐Openings via SN2 Reactions Under Basic and Acidic Conditions - Hansen - 2020 - European Journal of Organic Chemistry - Wiley Online Library